Table Of Content
Companies cannot afford such waste and expense if they are to survive. There are many design analysis tools and product testing methods available to designers and manufacturers. Most companies are very diligent about performing design analysis and verification testing.
Reporting
As previously discussed in the definition section, design verification involves the identification of specific requirements that pertain to the functionality, performance, usability, and safety of the device. This verification process aims to demonstrate that each of these identified requirements is effectively addressed. To facilitate the design verification process, it is imperative to develop a comprehensive test plan for each specific requirement. These test plans outline the procedures and methodologies to be followed in order to validate and verify the design outputs. The DVP&R format allows for complete and concise documentation of the analysis and verification activities completed during a new product introduction, design change or product re-certification. The Design Verification Plan and Report presents a clear picture understandable by the sponsor, stakeholders and team members, allowing all to understand the status of the analysis and verification testing.
Advantages of Design Validation and Verification
It just ensures you’ll spend even more time and energy trying to verify unclear design inputs or validate the poorly defined user needs. Design validation happens late in product development, but it’s based on the first step in the design controls process—defining your user needs. And as soon as you have your user needs, you can begin planning how you’ll validate them later on.
Design and Development Validation
Your documentation should include verification protocols, records, and reports. The protocols should document the detailed procedures, instructions, and parameters followed for each verification method, as well as any deviations or modifications from the original plan. Finally, verification reports should document the summary and analysis of the results, the status of each design output, any recommendations or actions taken, and any lessons learned from the process. During the design process, design verification plays a crucial role in ensuring that the design outputs align with the design inputs.
Growing Demand for Ausdia's Design Constraint Verification and Management Solution Leads to Expansion in Asia ... - Business Wire
Growing Demand for Ausdia's Design Constraint Verification and Management Solution Leads to Expansion in Asia ....
Posted: Tue, 12 Sep 2023 07:00:00 GMT [source]
Although it’s easy for professionals to incorrectly interchange the terms, "design verification" and "design validation" have very different meanings. Elena is a T-shaped UX Researcher with a varied cross-industry marketing background. She holds a BA in English as SLA, a Master of Science in Management and a professional UX Design Diploma.
This does not necessarily mean the first production unit, but it can. Once you decide what representative product you will build to prove the design, you fully test it to make sure that the product, as designed, will meet all the necessary requirements defined in the Design Inputs. You must take enough time to verify and validate a product to ensure you solve all issues. Everyone has time limits, but verification and validation should be priorities. Let’s learn more about the difference between design verification and validation, discover mistakes to avoid, and the benefits of both processes.
Benefits of design validation
A design verification traceability report shows test results and coverage for the requirements. Finally, reviews are completed and approved after each design verification activity. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. You can start thinking about how you’ll verify your design inputs as soon as you’ve defined them, and you may even be able to develop some of the tests you’ll use during product development. Each of these steps is important in the design process because they serve two distinct functions. Together, they ensure that the product designed will satisfy the customer needs, and the needs of the customer are one of the key focuses for ISO 9001 and improving Customer Satisfaction.
Additional Requirements
These requirements are outlined in various regulatory documents, including the FDA’s Quality System Regulation (QSR) and the Design Control Guidance for Medical Device Manufacturers. Medical device design verification is the process of testing to specification. It’s a technical process that verifies that your product does everything you claim it can do. For all devices and design controls, you should have clear, measurable specifications, called design inputs.
What do design verification and validation mean in medical device product development?
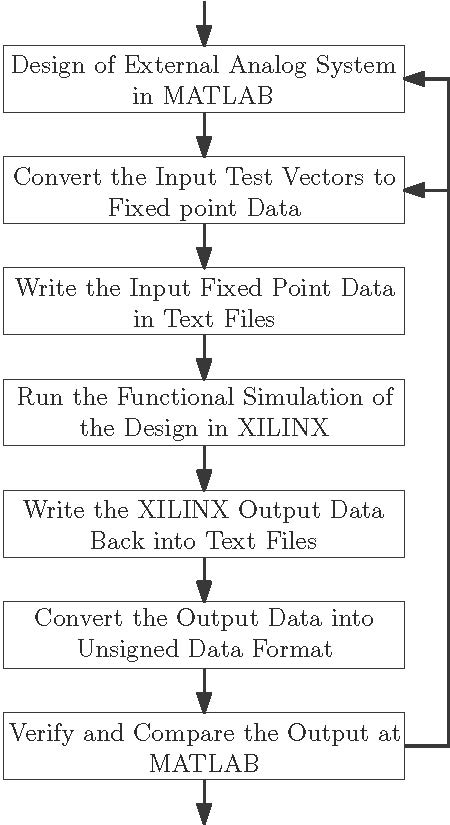
Some however do not have a good system or tool in place to organize and document the results. It also in some cases consists of folders of data understandable only by the design and test engineers. There is a tool currently in use in the automotive, heavy equipment and aviation industries that addresses this problem. This tool is relatively easy to understand and use and is very effective at organizing, describing, documenting and reporting the results of design analysis and product testing. You need to record your verification results, which provide objective evidence that your design outputs meet the design inputs and the intended use of your product.
Due to changes in the project schedule or availability of resources the testing dates and sample conditions can vary from the time the test is planned and actually performed. Design validation is of utmost importance as it is the best way to ensure that the design meets the needs of both the user and the business. UXers at Google revamped Google’s material design after conducting a very comprehensive design validation consisting of heuristic evaluations, usability tests, and peer reviews! The new system has been praised for its consistency across the board. A/B testing revolves around testing designs with a single variation to determine which performs better. This is a great method to use if your design validation objective is linked to a specific metric such as goal completion or a conversion rate.
But not to worry—in this guide, we’ll go through the basics of design verification and design validation, best practices you should follow, and common problems to avoid. Etienne Nichols is a Medical Device Guru and Mechanical Engineer who loves learning and teaching how systems work together. He has both manufacturing and product development experience, even aiding in the development of combination drug-delivery devices, from startup to Fortune 500 companies and holds a Project... There is an art to writing design inputs, and the way you go about it will be a major factor in how effective your verification process is. Probably the most misunderstood concept in the design requirements of ISO 9001, if not the entire standard, is the difference between Design Verification and Design Validation.

No comments:
Post a Comment